Dear Colleagues,
Paradigm Global Events is again proud to present our Orphan Drugs and Rare Diseases 2022 Americas – West Coast. It’s the 17th in the series of our Flagship tri-annual Orphan Drugs and Rare Diseases event, this congress will provide you with a comprehensive insight in establishing well-informed strategic decisions.
The global rare disease diagnostics market was valued at $45.18 billion in 2017 and is anticipated to reach $86.15 billion by 2025. Factors such as high incidence of rare diseases, presence of large numbers of research and development facilities for rare diseases, a significant number of rare disease registries, high diagnosis rare of rare diseases in countries of North America, and extensive investments in different therapeutic areas are driving the growth of the North America rare disease diagnostics market. However, the Asia Pacific region is expected to register the highest CAGR of 9.22% during the forecast period 2018-2025.
The growth of the global orphan drugs market is increasing at a rapid pace due to the growing rare diseases. With further growth is anticipated to meet the high unmet demand for more efficacious drugs with very few side effects. Although the number of people affected by rare diseases is considerably low, the return on investment is high due to the high cost of orphan drugs. Global collaboration is also likely to fuel growth. FDA recognizes the significance of orphan drugs in the treatment of rare debilitating, life-threatening diseases, therefore, supporting stakeholders to promote research and development in this area.
However, the industry is never without its challenges. Factors such as the lack of diagnosis, government policies, awareness and funding for R&D, along with long diagnostic delays, high initial investment that leads to higher per patient treatment cost, reimbursement uncertainties, and high cost of drug development are hindering the market.
Orphan Drugs & Rare Diseases 2022 Americas – West Coast will provide a unique platform for the convergence of stakeholders in the orphan drugs industry to discuss and network with top-tier government, hospitals, pharmaceuticals, biopharmaceuticals, non-profit organizations, orphan drugs developers as well as regional and local manufacturers. We are putting together an agenda that address the driving macroeconomic factors, policies, and issues that will steer the development of orphan drugs globally including commercialization, policies, reimbursement, pricing, and more.
We look forward to having you be part of the event!
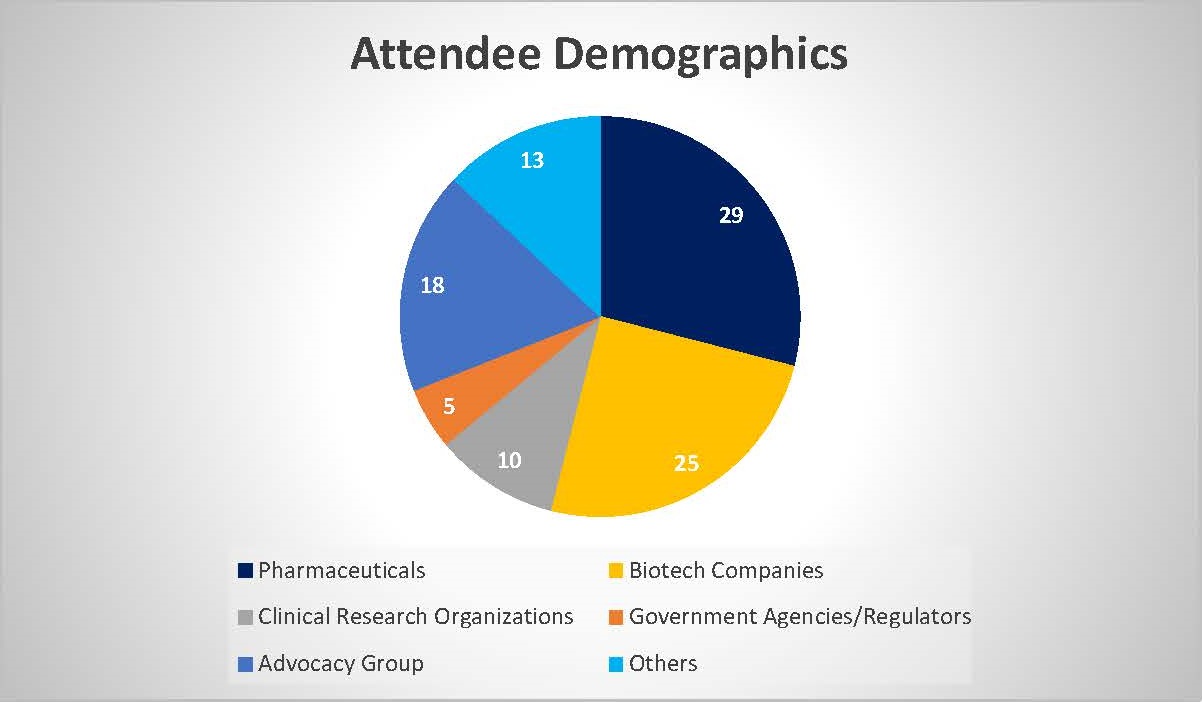
WHO WILL YOU MEET:
This congress is specially created for valued stakeholders in the Rare Disease community.
Presidents, Heads/Chiefs, Directors, VPs and Managers in the are of:
• Research and Development
• Personalised Medicine
• Regenerative Medicine
• External R&D Innovation
• Innovative Medicine
• Rare and Ultra-Rare Diseases
• Therapeutic Area Lead
• Cell and Gene Therapy
• Translational Science
• Molecular Geneticist
• Program Management
• Patient Advocacy Groups
• Patient Engagement
• Public Affairs
• Medical Affairs
• Regulatory Affairs
• Clinical Research Organizations
• Market Access
• Managed and Early Access
• Pricing and Reimbursement
• Health Economics Outcomes Research
• Commercial Development
• Investments and Funding
• Product Specialist
• Global Strategic Services
• Business Planning and Operations
• Speciality Pharmacies
• Academia
Gain Latest Insights On:
-
- The major market drivers in global rare disease diagnostic market
-
- Influencing factors that may affect market share
-
- Key developmental strategies carried out to stand out in this industry
-
- Finding innovative and alternative ways in funding the development of Orphan Drugs
-
- Key authorities facilitating development and approval of diagnostic products/services
-
- What Do Developers Look for When Looking for an Outsourcing Partner?
-
- Coming Together in Developing Orphan Drugs and Crossing Borders
-
- Trends and advancement in cell and gene therapy
-
- How Can the Developer and the Patient Assist in the Evolution and Development of Orphan Drugs?
-
- Access and Affordability: How Can This Continue to Improve?
-
- What Do Insurance Companies Think About Orphan Drugs? Will They Make Modifications to Their Policies to Support Patients with Rare Diseases?
To Sponsor/Exhibit
To Speak
To Register
sponsor@paradigmglobalevents.com
Tel. +44 203 567 1321
jocelynr@paradigmglobalevents.com
Tel. +44 203 567 1321
booking@paradigmglobalevents.com
Tel. +44 203 567 1321




